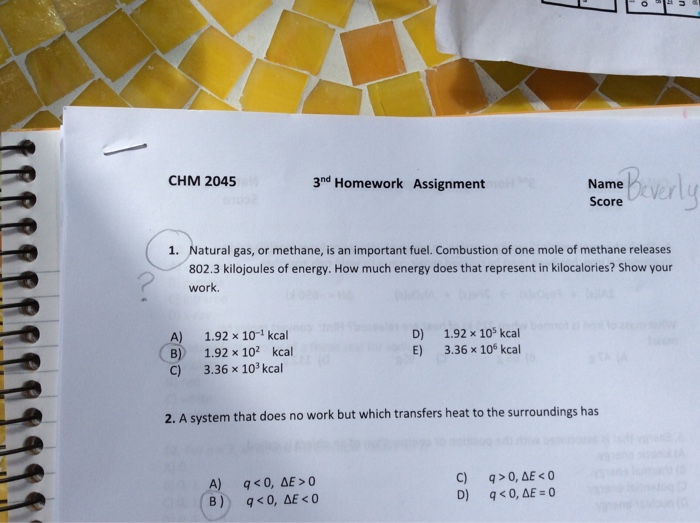
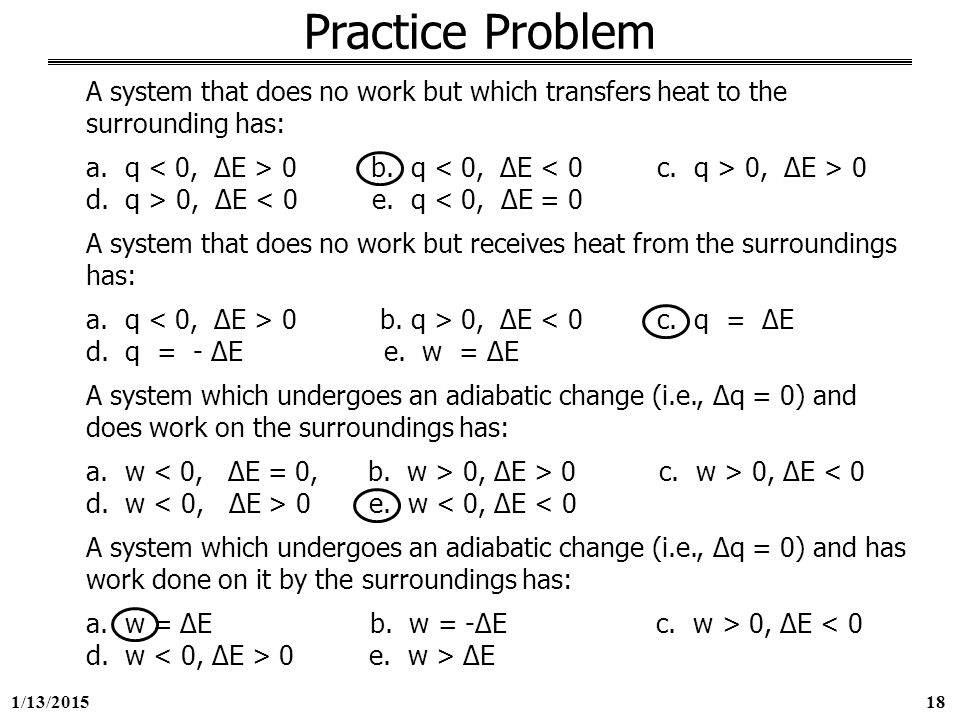
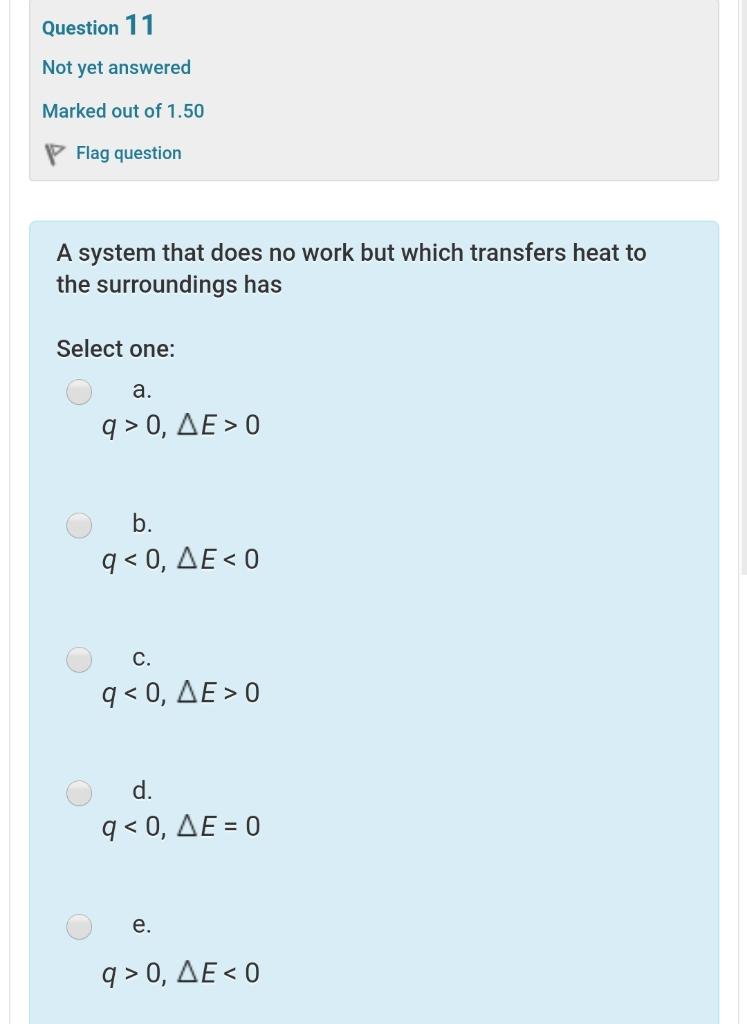
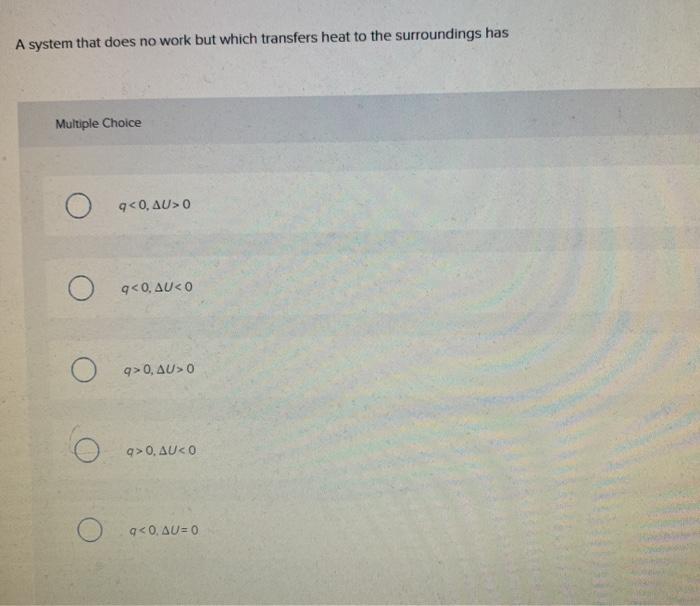
A System That Does Not Work But Which Transfers Heat To The Surroundings Has
A system that does not work but which transfers heat to the surroundings has. A system that does no work but which transfers heat to the surroundings has Select one. View the full answer. D q D E.
Heat transfer always occurs from the higher-temperature object or system to the lower-temperature object or system. A system that does no work but which transfers heat to the surroundings has. A system that does no work but which receives heat from the surroundings has.
A system that does no work but which transfers heat to the surroundings has. Q 0. Q 0.
W 0 ΔU 0 E. E 0 b q 0. A system that does no work but which receives heat from the surroundings has A q 0 D E 0.
A reaction or process in which heat is transferred to a system from its surroundings is endothermic. It can do work on the surroundings. W 0 ΔU 0 D.
W ΔU The dissolution of barium hydroxide in water is an exothermic process. Firstly differently known as an isocaloric process the adiabatic process is when theres no transfer of heat from or towards the fluid being worked on. Q 0.
Q 0 ΔE 0. Figure 19 a Here the soft drink has a higher temperature than the ice so they are not in thermal equilibrium.
A system that does no work but which receives heat from the surroundings has.
B q 0 D E 0. It can do work on the surroundings. A system that does no work but which receives heat from the surroundings has. The risk of spreading SARS-CoV-2 the virus that causes COVID-19 through ventilation systems is not clear at this time. W 0 ΔU 0 D. D q D E. Q 0 ΔE 0. Heat transfer always occurs from the higher-temperature object or system to the lower-temperature object or system. An isolated system is one that exchanges no matter heat or work with the surroundings so that the mass and total energy of the system remain constant over time.
A system that does work on the surroundings with no heat change ie q 0 has. Besides adiabatic would indicate impassable if defined actually. E w D E. W 0 ΔU 0 B. B q 0 ΔU 0. Heat transfer always occurs from the higher-temperature object or system to the lower-temperature object or system. A reaction or process in which heat is transferred to a system from its surroundings is endothermic.




























Post a Comment for "A System That Does Not Work But Which Transfers Heat To The Surroundings Has"